Potassium Dichromate Colour Change
Here is the equation. H 2 SO 4 green.
What Is The Precipitate In Potassium Dichromate Sulfuric Acid And Potassium Iodide Quora
Reaction to form hydrogen chromate ions or dichromate ions affecting the accuracy of the end point.
. The combustion reaction of candle wax is characterised by a change in state from solid to liquid and gas because the wax is a solid water formed by the. Mohrs salt Titration with Potassium Dichromate. It is a good idea to first carry out a rough titration in order to become familiar with the colour change at the end point.
If the potassium dichromate solution changes colour from orange to green then an oxidation reaction has taken place with a primary or secondary alcohol. Bind dyes on fabrics by forming a coordination complex with the dye which then attaches to the fabric or tissue. The oxidation of the alcohol to an aldehyde is indicated by the colour change of the dichromate solution as it is reduced from the orange colour of Cr 2 O 7 2 to the green of chromiumIII ions Cr 3.
A primary alcohol will form an aldehyde and a secondary alcohol will form a ketone. You should check the result as soon as the potassium dichromateVI solution turns green - if you leave it too long the Schiffs reagent might start to change colour in the secondary alcohol case as well. 4K 2 Cr 2 O 7 4K 2 CrO 4 2Cr 2 O 3 3O 2.
A mordant or dye fixative is a substance used to set ie. Chemical Properties of Potassium Dichromate. When the solution of K 2 Cr 2 O 7 reacts with an alkali ionic salt a yellow solution is obtained because of the potassium.
Then the chromic ions oxidize the glycerol and reduce into chromous ions by giving a blue colour to the solution in the presence of nitric acid. Tollens reagent or Fehlings solution can then be used to. Aim To prepare M20 solution of Mohrs salt and.
The chromiumIII oxide is then reduced to chromium with aluminum metal. Then add a few drops of 5 potassium dichromate solution. Sodium nitroprusside Complex of Purple colour 3.
K 2 Cr. Take 2-3 ml of a sample in a test tube. The titration of potassium permanganate with respect to the mohr salt is a major example of redox titration.
There are three indicators that may be used for the titration of Fe 2 with K 2 Cr 2 O 7. The sodium dichromate is filtered out and reduced with carbon to produce chromiumIII oxide sodium carbonateand carbon monoxide. In the potassium dichromate solution Cr 2 O 7 2-ions in orange colour are in equilibrium with CrO 4 2-ions in yellow colour.
B What mass of chromium in kg could be pre-pared from the 2003 world production of chromite. In this glycerol and dichromate ions react to give a brown colour to the solution. H 2 SO 4 SO 2 gas is evolved which is suffocating with the smell of bur ning sulphur.
Although mordants are still used especially by small batch dyers it has been largely displaced in industry by directs. Introducing heat to K 2 Cr 2 O 7 decomposes it into potassium chromate K 2 CrO 4 and produces O 2 gas. Unlike permanganate dichromate titrations require an indicator.
2KMnO 4 3H 2 SO 4 K 2 SO 4 2MnSO 4 3H 2 O 5O Or MnO 4 8H 5e Mn 2 4H 2 O. Iodimetric and Iodometric Titrations. A Write balanced equations for each step.
Iii Change in state of substance. In this reaction. Search all type Industrial Raw Materials its Sellers in India Browse by Industry Category Send Inquiry Get Multiple Sellers Responses List Raw Material Products PlasticsPolymer PharmaceuticalsDrug Chemicals Paints PesticidesInsecticide MetalsSteel OreMineral YarnFibre RubberElastomer Construction CeramicsGlass Fertilizers.
A common method for Chemical Oxygen Demand analysis involves using a strong oxidizing chemical potassium dichromate Cr 2 O 7 2- to oxidize the organic matter in solution to carbon dioxide and water under acidic conditions. Enter the email address you signed up with and well email you a reset link. Na 2 SO 3 H 2 SO 4 Na 2 SO 4 H 2 O SO 2 The gas turns potassium dichromate paper acidified with dil.
These are diphenylamine diphenylbenzidine and diphenylamine sulfonate. Test for Sulphite ion SO2 3 a On treating sulphite with warm dil. Common sodium potassium and ammonium compounds are soluble all nitrates are soluble common chlorides are soluble except those of silver and leadII common sulfates are soluble except for those of barium calcium and leadII common carbonates are insoluble except for those of.
It is maintained with the use of dilute sulphuric acid. It may be used for dyeing fabrics or for intensifying stains in cell or tissue preparations. At work request a material safety data sheet to help identify alternatives that are safe hence avoiding contact with material containing chromates.
Cr 2 O 7 2- H 2 O 2CrO 4 2- 2H Orange-red Yellow. Avoid all of these. 234 know the general rules for predicting the solubility of ionic compounds in water.
The action of the indicator is analogous to the other types of visual color titrations in the close proximity of the end-point in oxidation-reduction titration. The chemical reaction between sulphur dioxide gas and acidified potassium dichromate solution is characterized by a change in colour from orange to green. If no colour change is observed then the alcohol was a tertiary alcohol.
The Mohr titration is sensitive to the presence of both chloride and bromide ions in solution and. In this titration the potassium permanganate is used as an oxidizing agent. Because of the colour change to the acidified potassium dichromateVI solution you must therefore have a secondary alcohol.
Butanol is oxidised by sodium dichromate Na 2 Cr 2 O 7 acidified in dilute sulphuric acid to form the aldehyde butanal. The colour change for all three indicators is green to violet and the standard electrode potentials are all ca 078 V. This colour change of orange to yellow and yellow to orange can be explained on the concept of equilibrium of the two ions.
Cr 2 K 2 0 7 the hexavalent form of chromium Cross-reactions.

The Colour Change Of Acidified Potassium Dichromate The Real Chemist Youtube
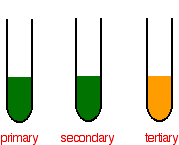
14 7 Determining Alcohol Classifications In The Lab Alternate Reactions Chemistry Libretexts

Potassium Dichromate Test For Alcohols Stock Image C040 2657 Science Photo Library
Content Redox Chemistry Inside The Breathalyzer The Alcohol Pharmacology Education Partnership
No comments for "Potassium Dichromate Colour Change"
Post a Comment